Register to download more information about ellacor 2.0.
You are about to leave the ellacor site and be transferred to an independent medical portal.
ellacor with Micro-Coring Technology is the first and only non-surgical solution to remove sagging skin without surgery.
As patients’ expectations continue to rise, you need a novel solution that can deliver better results. Developed alongside physicians, ellacor provides value to you and your practice while delivering the results your patients expect, treating moderate to severe wrinkles in the mid to lower face without surgery.
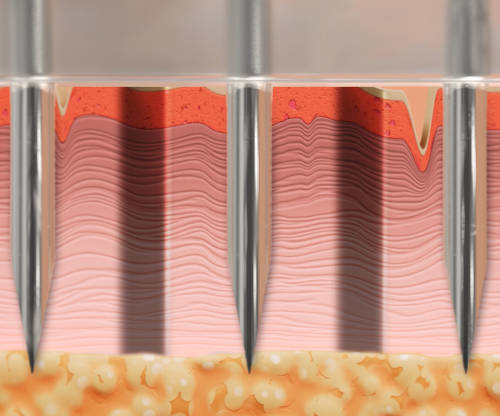
Proprietary excision mechanism removes full thickness micro-cores of dermal and epidermal tissue.
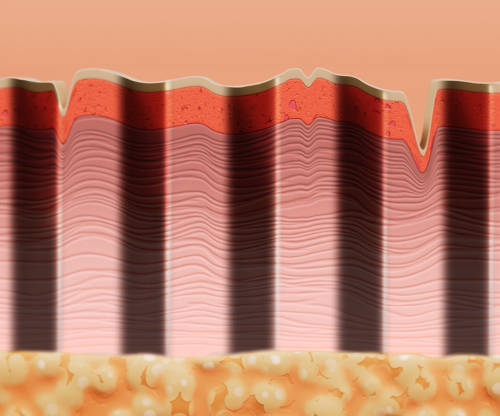
With no heat used or produced during treatment, ellacor does not cause thermal injury.
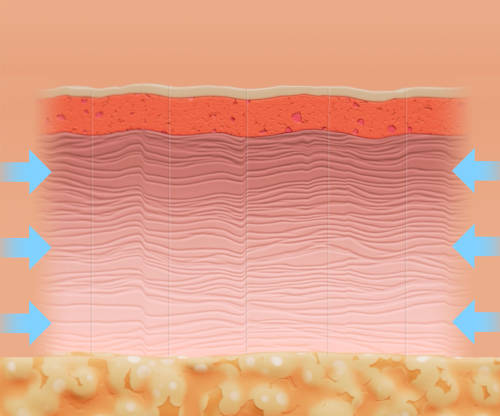
The skin naturally realigns along the relaxed skin tension lines, resulting in improved skin architecture.2
The ellacor System with Micro-Coring Technology was evaluated in more than 200 patients.
Get the full story on how ellacor can help elevate your practice.
The ellacor® System with Micro-Coring® technology is indicated for use by medical professionals for the treatment of moderate and severe wrinkles in the mid and lower face in adults aged 22 years or older with Fitzpatrick skin types I-IV.
Your medical professional will discuss the following side effects with you prior to treatment. Proper pre- and post-treatment care reduces the risk of these side effects; however, some conditions may or may not resolve over time. Side effects associated with this procedure may include: redness, swelling, bruising, burning, dryness, roughness, tightness/pulling of skin, crusting, pain/discomfort, tenderness, tingling, bleeding, numbness, skin peeling, or circular marks on skin.
Other side effects not commonly observed with this procedure may include: itching, hyper/hypo pigmentation, hematoma, infection, scarring, skin irregularities, skin necrosis, uneven appearance of the treated regions (left and right sides of face), or anesthesia toxicity (anesthesia-related complications may include allergic reaction and possibly death).
The ellacor® procedure should not be used for the treatment of areas of skin with dermatosis (e.g. skin tumors, keloids or in case of predisposition to keloids, solar keratosis, warts, or birthmarks), area within the bony orbital rim; mucous membranes; areas where silicone or synthetic material is implanted.
The ellacor® procedure should not be used on patients that are pregnant or nursing mothers, suffering from open wounds, sores, or irritated skin in the treatment area, have an allergy to stainless steel or allergy to topical, oral, or injected medications or preparations that may be used during the procedure, such as petrolatum, lidocaine, bupivacaine, chlorhexidine, or povidone-iodine. Patients that have a history or presence of any clinically significant bleeding disorder, have dermatological or autoimmune conditions that may affect the treatment outcome; these may include, but are not limited to: actinic keratosis, raised nevi, rosacea, melasma, active acne, cutaneous papules/nodules, active inflammatory lesions, dermatitis, psoriasis, cellulitis, urticarial folliculitis, acute inflammatory phase of scleroderma, rheumatoid arthritis, eczema, psoriasis, allergic dermatitis, collagen disorders, or lupus should not be treated with the ellacor® procedure. Additionally, patients that have systemic infections or acute local skin infections such as Hepatitis disorders type A, B, C, D, E or F or HIV infection, take a high dose of anti-coagulants or blood-thinning substances (e.g. aspirin, nonsteroidal anti-inflammatory drugs, NSAIDs, warfarin, heparin, acetylsalicylic acid during the previous fourteen (14) days), are on courses of chemotherapy, high-dose corticosteroid use, or radiation in the treatment area, have undergone plastic surgery of the face within the last twelve (12) months or have any facial surgical scars less than twelve (12) months old, have undergone injections of dermal fillers, fat, or botulinum toxin, as well as any minimally invasive/invasive skin treatment in the treatment area during the previous six (6) months, or have scars less than six (6) months old in the treatment area should not be treated with the ellacor® procedure.
Appropriateness for treatment is based on the clinical assessment of the patient by the treating physician. Use caution when treating patients with the following conditions or taking the following medications: